Batch Record Review for GMP Products
Batch Record Review and Product Release
GMP Batch Record Review
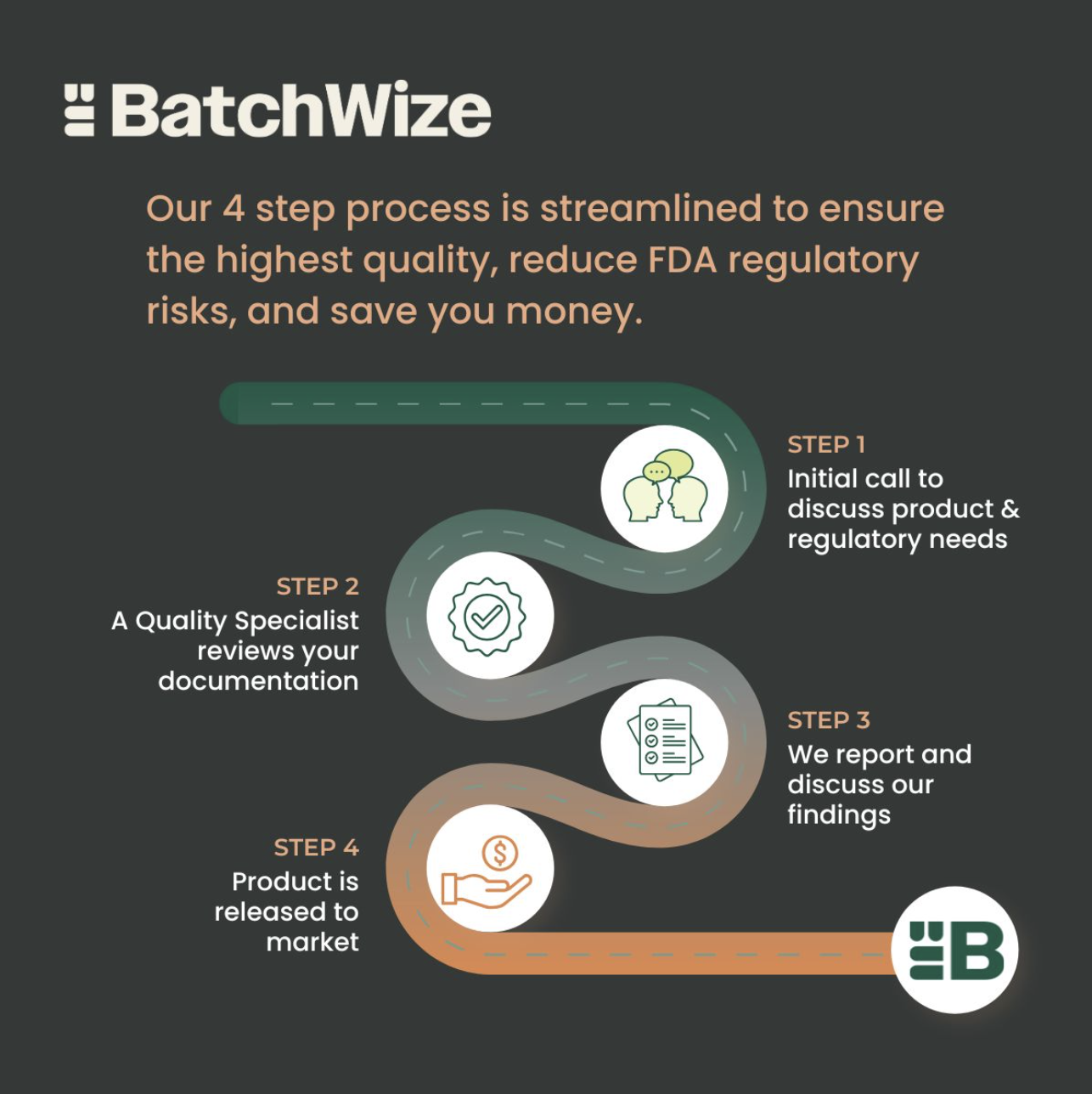
Need support with batch record review and product release? Here's what to expect from BatchWize.
BatchWize provides a cost effective review of manufacturing batch records for medical device, pharmaceutical, food & beverage and cosmetics companies. Our certified quality reviewers focus on product quality, minimizing product waste, and creating audit-ready GMP records.
The Importance of Batch Records and How We Can Help with Batch Record Review
In FDA-regulated industries, batch records are the cornerstone of quality and compliance. These records document every step in the manufacturing process, ensuring that products are produced consistently, meet predefined specifications, and comply with regulatory requirements.
Whether you’re manufacturing pharmaceuticals, medical devices, food, or cosmetics, maintaining accurate and complete batch records is critical—not just for compliance but for ensuring product safety and quality.
Why Are Batch Records So Important?
Batch records serve as both a roadmap and a record of what happened during production. Here’s why they’re essential:
Regulatory Compliance: FDA and other regulatory bodies require thorough documentation to ensure products are manufactured according to approved processes.
Product Quality and Safety: Batch records verify that every product meets quality standards, reducing risks of recalls or safety issues.
Audit Preparedness: Complete and accurate batch records are critical during inspections and audits. Missing or incomplete records can lead to costly observations, warning letters, or fines.
Traceability: In the event of a deviation, batch records provide the traceability needed to identify root causes and take corrective actions.
Efficiency: Well-maintained batch records streamline production and prevent costly delays caused by errors or incomplete documentation.
The Challenges of Batch Record Review
Despite their importance, batch record review can be a time-consuming and complex process. Common challenges include:
Human Error: Manual documentation errors can lead to inconsistencies or missing information.
Volume of Records: Organizations with high production volumes may struggle to keep up with timely reviews.
Regulatory Complexity: Understanding and adhering to evolving FDA requirements can be daunting.
Resource Constraints: Limited staff or expertise can delay reviews and approvals.
How Our Consulting Services Can Help
If batch record review is becoming a bottleneck in your operations, our expert consulting services are here to support you. We specialize in:
Comprehensive Batch Record Review: Our consultants will thoroughly review your records for accuracy, completeness, and compliance with FDA and industry standards.
Process Improvement: We’ll identify inefficiencies in your documentation process and recommend improvements to streamline review timelines.
Gap Analysis: Our team can pinpoint gaps in your batch records and provide corrective strategies to ensure they meet regulatory requirements.
Training and Support: We offer tailored training programs to help your team improve documentation practices and stay compliant.
Audit Preparation: Our experts will ensure your batch records are audit-ready, reducing the risk of observations or citations during inspections.
By partnering with us, you’ll save time, reduce errors, and ensure your batch records meet the highest standards of quality and compliance.
Don’t Let Batch Records Hold You Back
Batch records are too important to leave to chance. Whether you need help clearing a backlog, preparing for an audit, or improving your documentation process, our consulting services are here to help.
👉 Contact us today to learn how we can optimize your batch record review process and support your compliance goals.
Still have questions? Send us an email: Team@BatchWize.com