Welcome to Our Blog!
Our blog is your go-to destination for insights, updates, and expertise on FDA regulated products. Whether you're looking for practical tips, the latest trends, or in-depth discussions, we’ve got something for everyone.
At BatchWize, we’re passionate about sharing knowledge and helping you stay informed and inspired. Dive in, explore our posts, and feel free to join the conversation by reaching out—we’d love to hear from you.

Part 2- Optimizing Pharmaceutical Product Release featured in GxPLifeline
Optimizing Pharma Product Release: Part 2
How can you make the pharma product release process faster, smoother, and more cost-effective? In Part 2 of our blog series, we explore:
Key planning strategies to avoid delays and deviations.
How electronic systems (eQMS & EBR) streamline data and compliance.
Tips for partnering effectively with CMOs while staying FDA-compliant.

Part 1- Pharmaceutical Product Release featured in GxP Lifeline!
The part 1 of this two-part series on pharmaceutical product release explores key foundational concepts, starting with the definition of product release and its critical role in guaranteeing the safety and effectiveness of drugs.

The Risks of Overdue CAPAs and How to Prevent Them
Overdue CAPAs may result in unresolved quality issues, which can affect product safety and effectiveness. Learn about the risks of overdue CAPAs and how to prevent them.

BatchWize Focuses on Bridging Innovation at Austin MedTech Connect
Thank you, Austin Medtech Connect, for hosting an inspiring summit!
Batch Record Review for GMP Products
In FDA-regulated industries, batch records are the cornerstone of quality and compliance. These records document every step in the manufacturing process, ensuring that products are produced consistently, meet predefined specifications, and comply with regulatory requirements.

BatchWize Shares Their Experience With Electronic Quality Management Systems (eQMS)
MasterControl's GxP Lifeline Blog is a wealth of information and BatchWize is honored to have contributed to the topic of Quality Managements Systems (eQMS), and Electronic Batch Records.

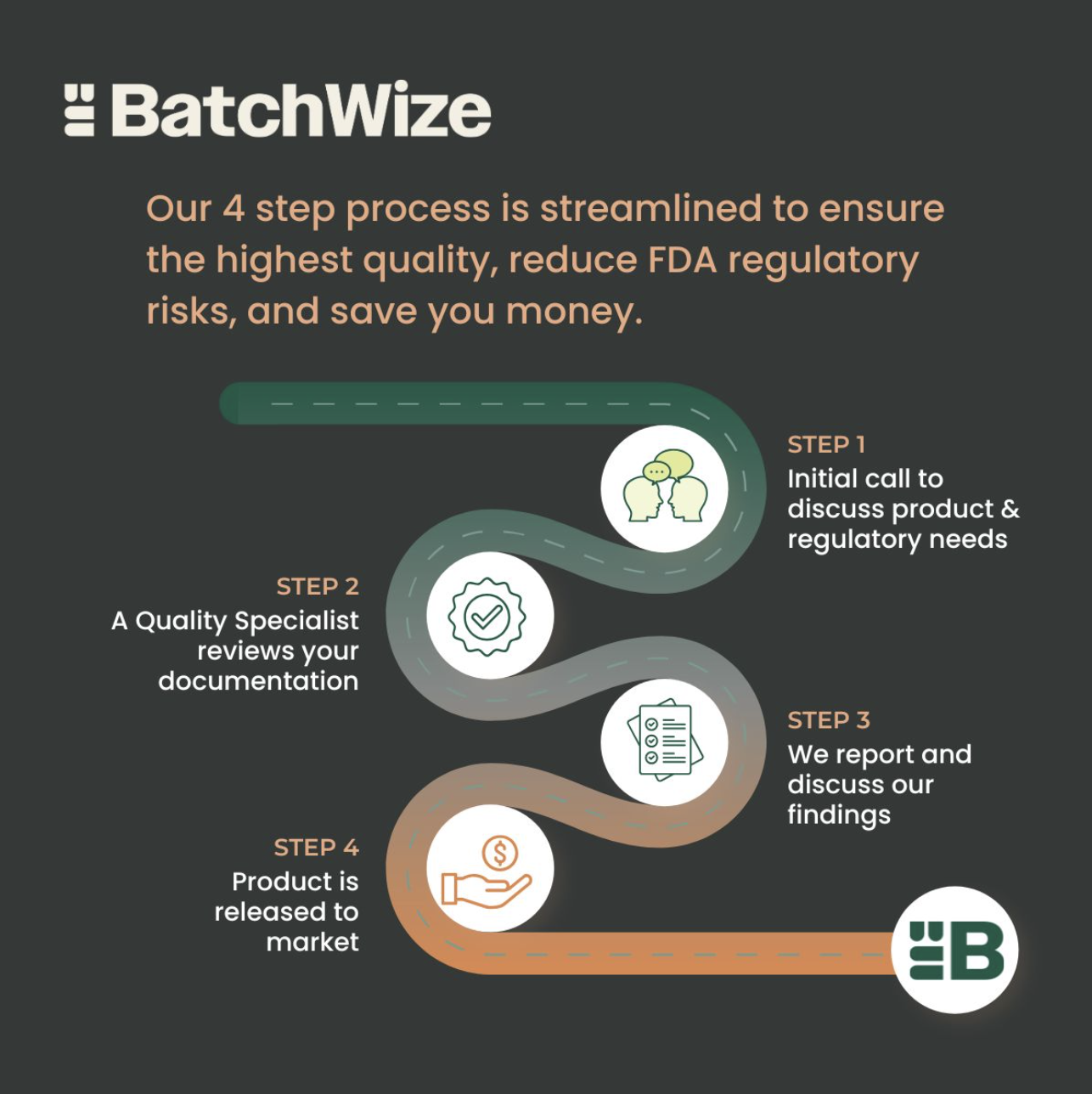
BatchWize Services: GMP Product Release & Quality Support
At BatchWize, we have the ability to customize our services to meet our client’s needs. Some clients benefit from a full and thorough manufacturing batch record review, some clients need help reviewing nonconformances, and others want a closer look at their manufacturing data using trending and process visualization tools.

Keeping Batch Records for Herbal Products
Making small amounts of herbal products for friends and family can be quite different than making herbal products on a large scale for commercial use. When deciding to make a product for sale, it is always a good idea to keep detailed records, also known as Batch Records or Manufacturing Batch Records. But what exactly is a Batch Record? Why is it important? And why would I need one? Let’s take a closer look.

Batch Records 101: What You Need to Know
Manufacturing Batch Records are essential documents that provide a detailed account of the production process for a specific batch of a product. Batch Records are critical for manufacturers and license holders in regulated industries like Pharmaceuticals, MedTech, Food & Beverage, Supplements, and Cosmetics.

Trusted Partners with MasterControl
BatchWize is thrilled to announce that we are a MasterControl Trusted Partner! MasterControl Inc. is a leading provider of cloud-based quality and manufacturing software for life sciences and other regulated industries. We will be leveraging MasterControl for electronic record review and product release of FDA Regulated Products.

The Value of Manufacturing Record Review
Manufacturing Batch Record Review provides valuable benefits, especially within regulated industries such as pharmaceuticals, biotechnology, food, wellness and medical devices. The primary values and benefits of a thorough manufacturing batch record review include:

New Website Launch
We are pleased to announce the launch of our new brand & website! The BatchWize website is a reflection of our company, our values, and the type of work we do. Our new brand reflects high quality services that are accessible to all manufacturers. BatchWize is Batch Release Made Easy!

A letter from Rebecca Waterbury, CEO
BatchWize™ is more than a company, it is a movement. BatchWize™ represents the quality of work that we hope to produce and the mark that we want to make in our community. Our focus is simple: we’re helping our customers get quality products to market, faster.
